REVIEWS
Hear what the diabetes community
is saying about the Q-Pad
Hear what the
diabetes community
is saying about the Q-Pad
HARNESS YOUR HEALTH
How it works.
All from Home.
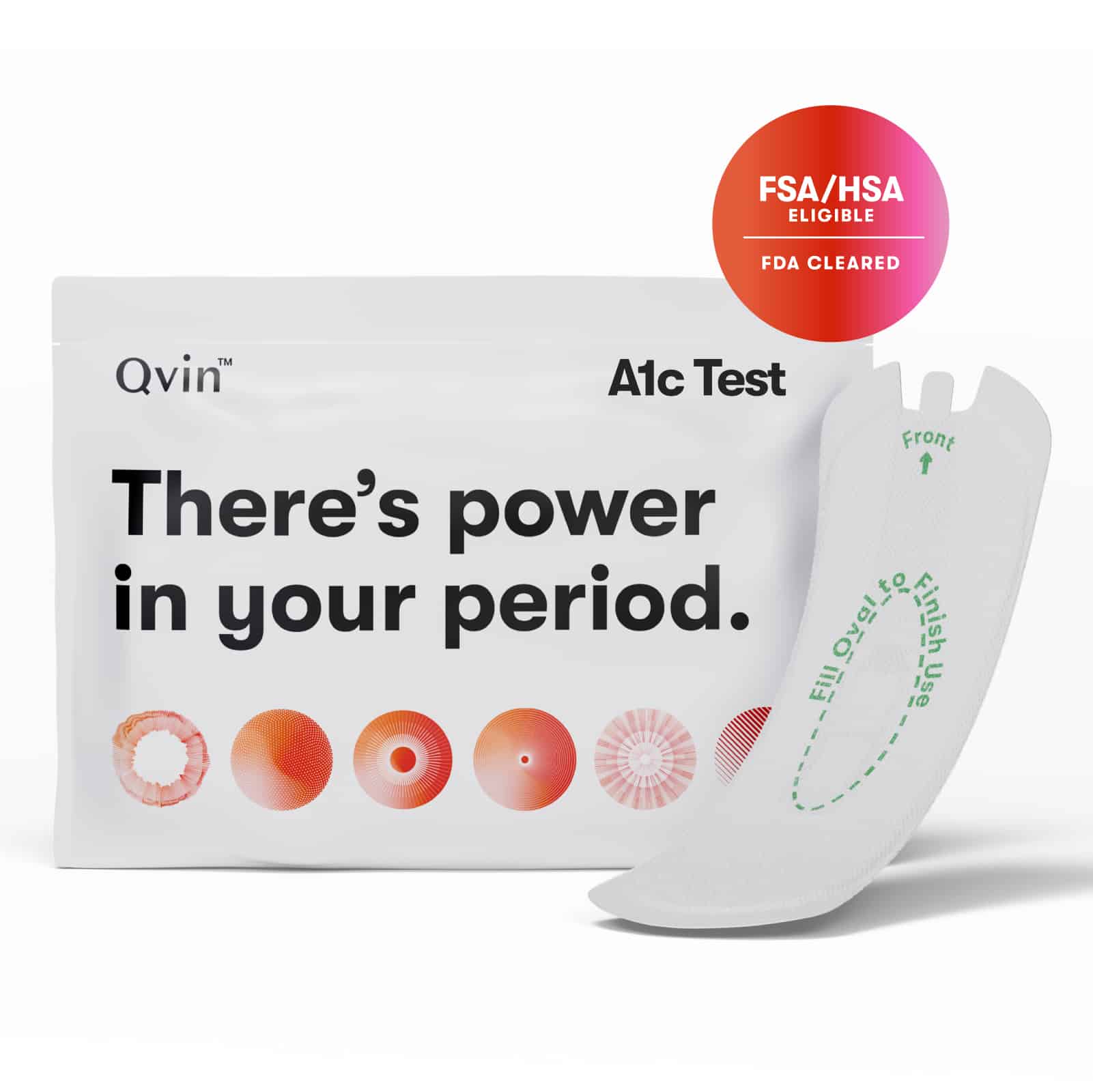
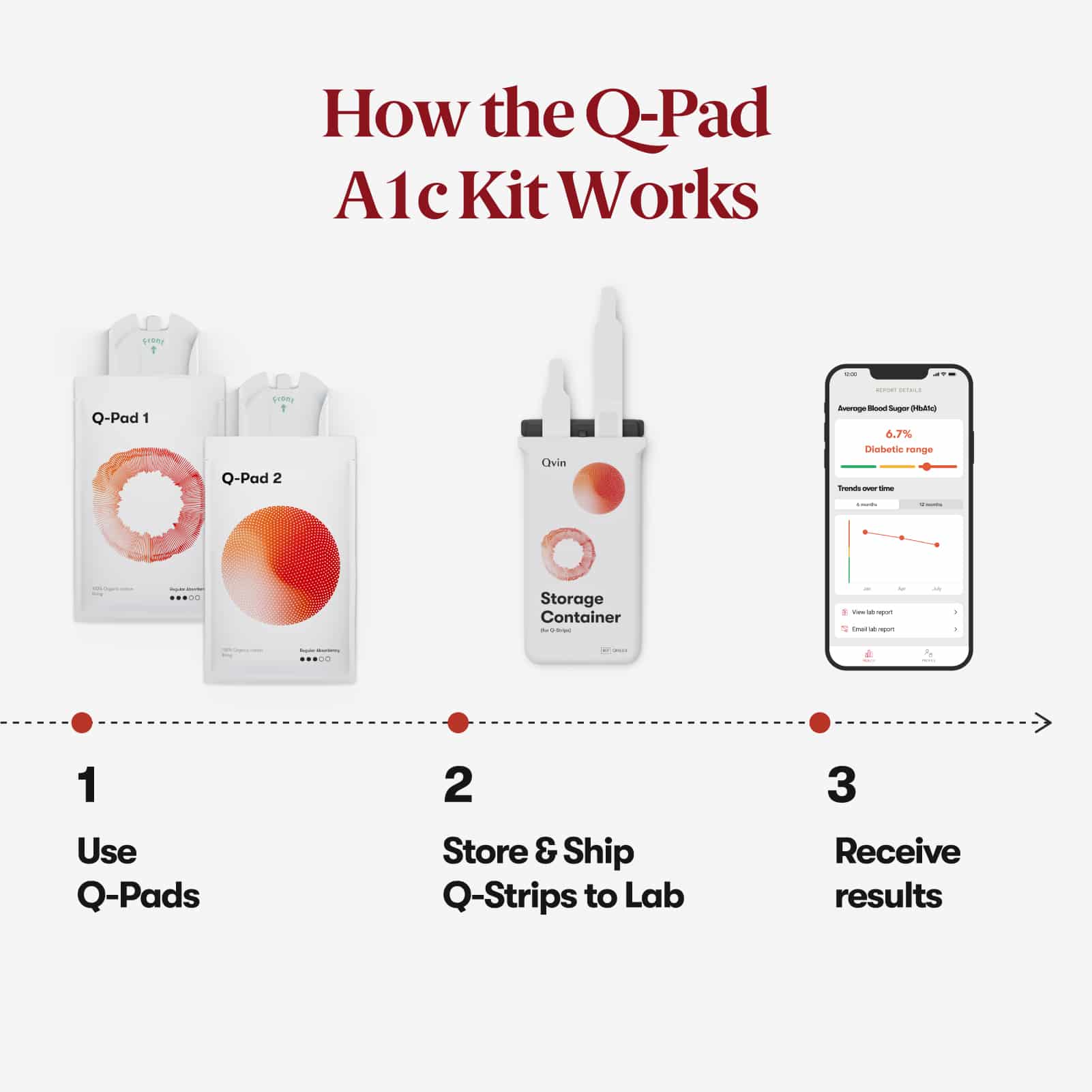
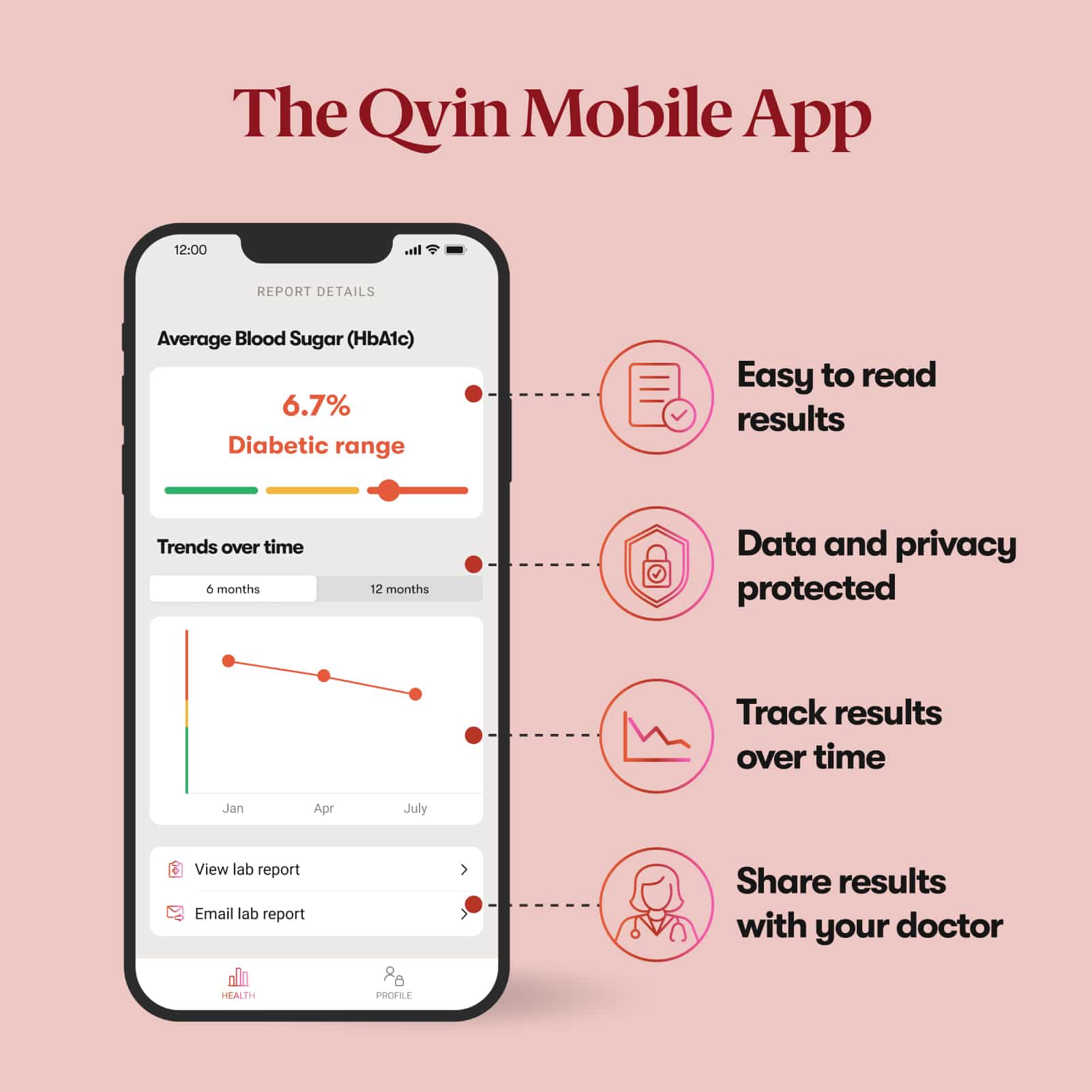
Use two Q-Pads during your monthly cycle. Mail both collection strips to the lab in pre-paid packaging and you will receive your A1c lab report in our app. Results in 5 - 10 days after shipping.


1. Use Q-Pads
Use both Q-Pads on your heaviest
period days.

2. Store & Ship
After use, remove the Q-Strip from
the Q-Pad and place into the
Storage Container.

1. Use Q-Pads
Use both Q-Pads on your heaviest
period days.
Preparation
Things to know.
Qvin is primarily an online service. A smartphone with internet access is recommended for optimal use of our service and to view your lab reports.
Use on your heaviest period days, and days 2- 4 of the menstrual cycle.
These reports are not intended to diagnose, treat, or prevent any disease. The Qvin laboratory is CLIA Certified and meets state and federal regulations.
OUR APPROACH TO HEALTH
Built on Scientific Standards and Data Protection
Built on
Scientific Standards
and Data Protection
Science First
We have numerous papers published in peer-reviewed medical journals, including scientific publications on venous (ie. blood from veins) blood compared to menstrual blood for diagnostic purposes. All published data uses our Q-Pads.
FDA-Cleared
The Q-Pad A1c Test has met FDA scientific requirements for FDA-clearance in people with diabetes. See our A1c Test Accuracy Data.
CLIA Certified Lab
Our lab is CLIA Certified (Clinical Laboratory Improvement Amendments). This means it has to meet the high quality standards of the federal statute and submit to regular inspections.
Secure Data
Qvin takes your privacy seriously. We use the latest security standards and encryption to ensure your data is stored securely, require Two Factor Authentication (2FA) and support biometric sign in for the Qvin app, anonymize and de-identify data to ensure your privacy, and under no circumstances do we ever sell your data.