DIAGNOSING CONDITIONS
Undestanding
Cervical Cancer
Cervical cancer occurs when abnormal cells in the cervix grow uncontrollably. While it is entirely preventable and highly treatable when detected early, many cases go undiagnosed due to barriers to screening and delays in care.
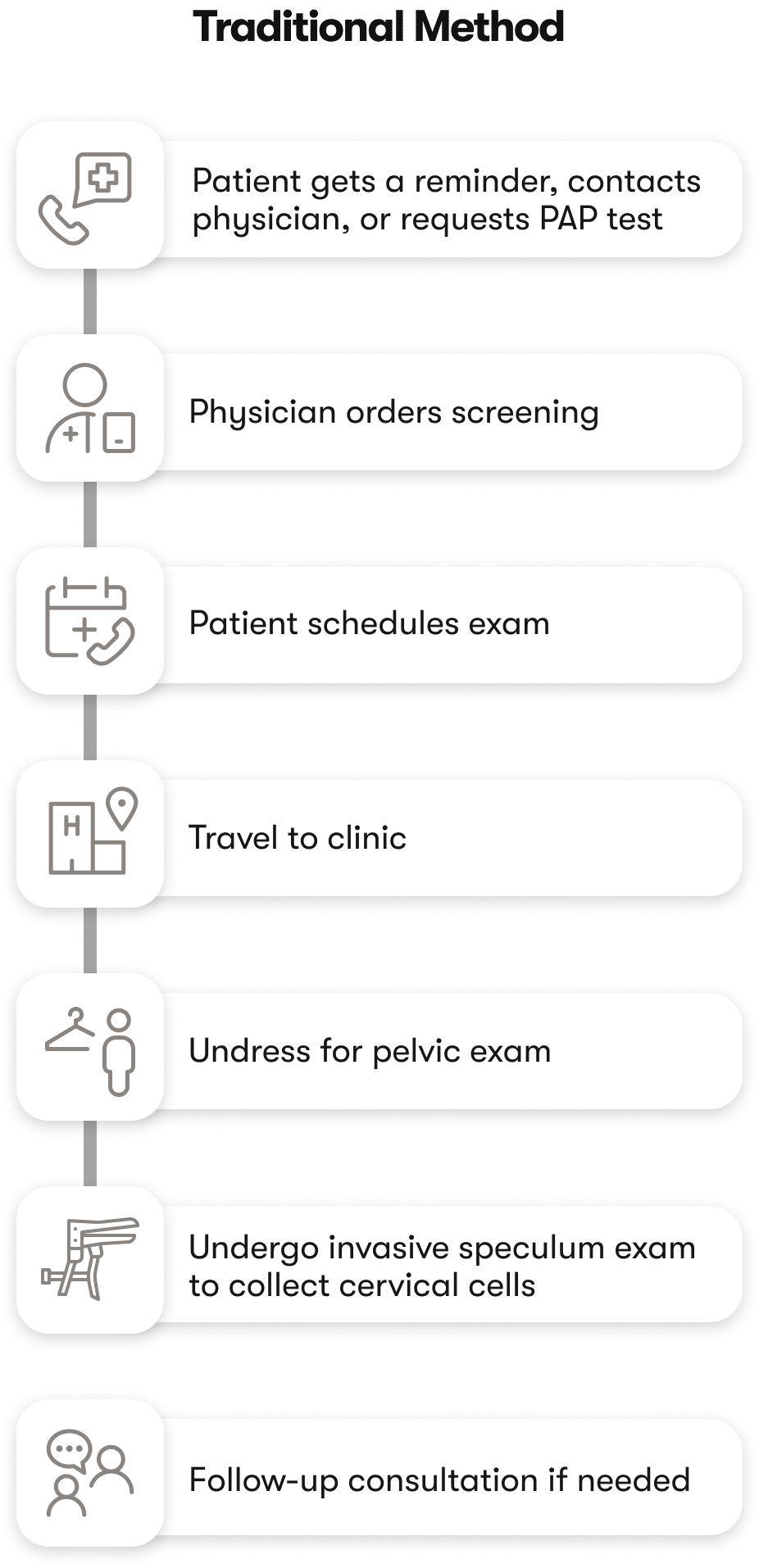
HOW THE CURRENT SYSTEM IS FAILING WOMEN
Early Detection Is Crucial for Cervical Cancer Treatment
Cervical cancer often develops silently, with early stages showing no symptoms. Regular screening is essential, but many individuals may not notice symptoms until the cancer reaches an advanced stage.
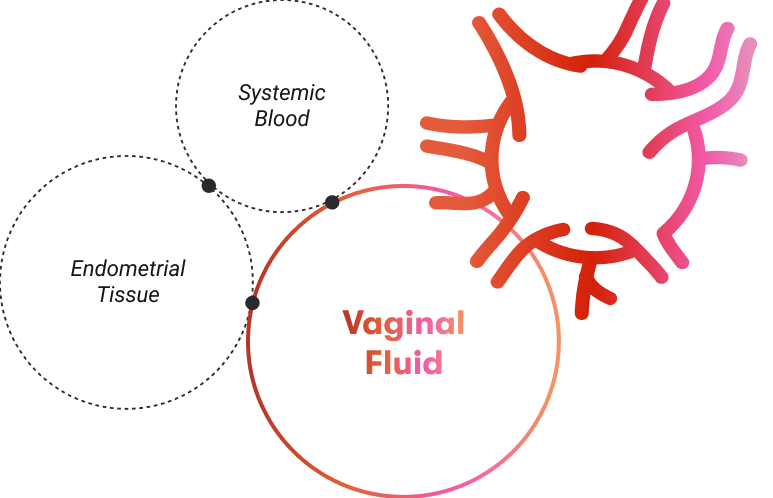
Menstrual blood can help women detect cervical cancer earlier
Cervical cancer is the fourth most common cancer in women globally.
New cases of cervical cancer are diagnosed worldwide every year.
Lives are lost to cervical cancer globally each year.
Of cervical cancer cases occur in low- and middle-income countries.
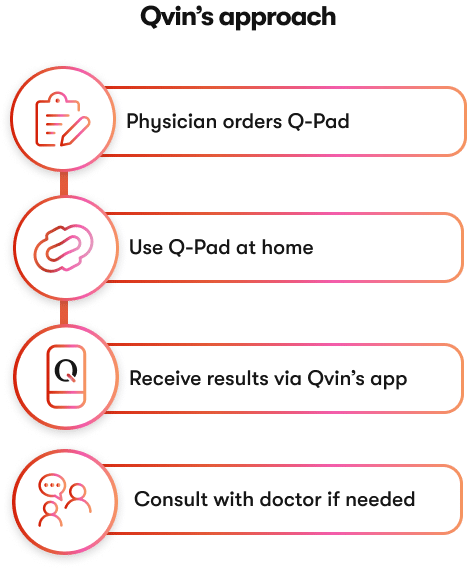
Qvin Simplifies
Cervical Cancer Screening
Partner with Qvin
THE PROBLEM
Approximately $2.3 billion was spent on
cervical cancer treatment in the U.S. alone in 2020.
THE SOLUTION
Qvin makes screening of cervical cancer more accessible, increasing early detection, reducing costs, and saving lives.
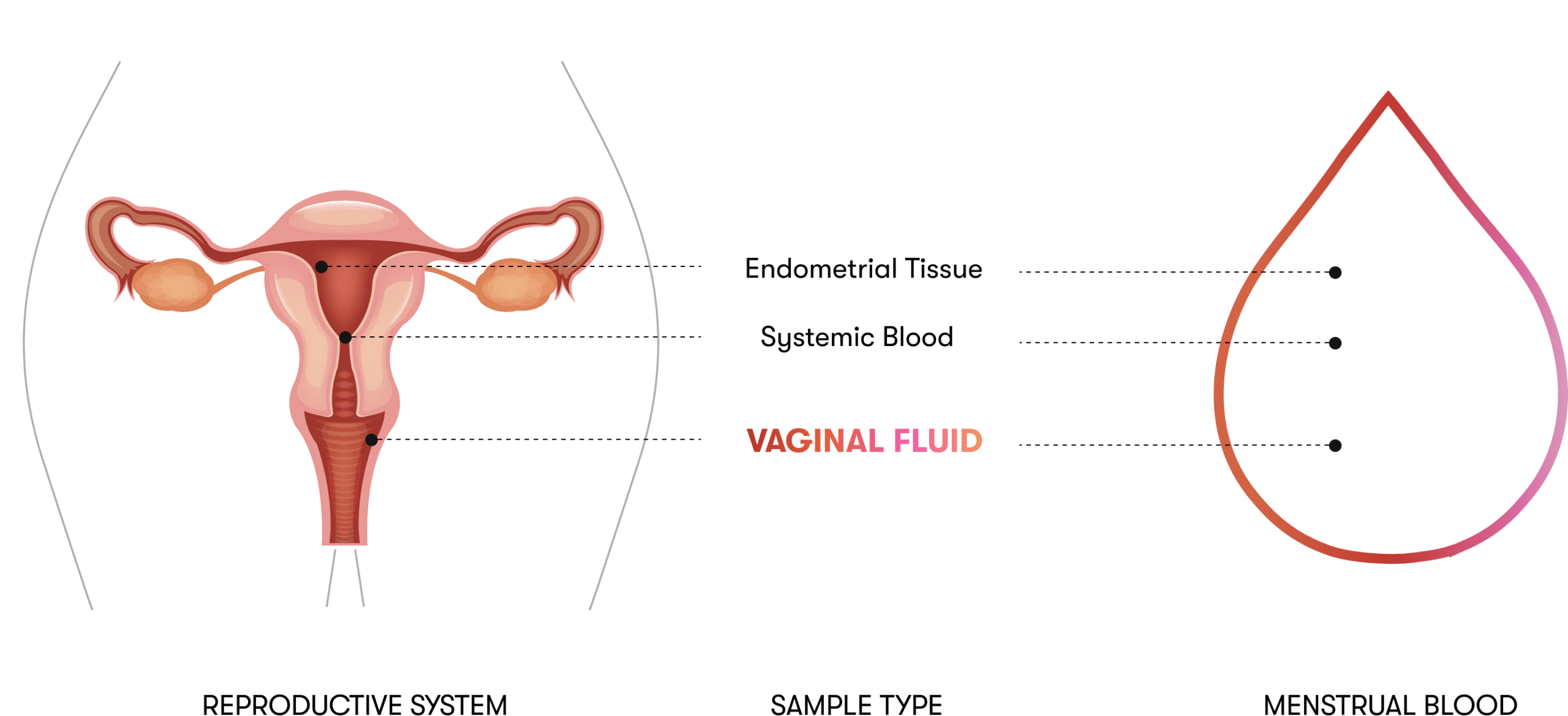
Rationale for the Q-Pad for Cervical Cancer
Menstrual blood (MB) is a non-invasive, self-collected sample containing exfoliated cervical and endometrial cells, making it a clinically viable medium for detecting high-risk human papillomavirus (HR-HPV). Studies have shown strong diagnostic performance: Wong et al. reported 82.8% sensitivity and 93.1% specificity using PCR-based detection [1]; Zhang et al. achieved 97.7% sensitivity and 92.7% concordance with cervical samples via next-generation sequencing [2]; and Lee et al. found 87.5% agreement in high-grade lesion cases when MB was collected on day one of menstruation [3]. In a 2022 Stanford University Hospital study, Naseri et al. evaluated the Q-Pad—a modified menstrual pad with a dried blood spot (DBS) strip—using the Roche Cobas HPV assay, showing 94% concordance with clinician-collected samples and 100% agreement in CIN2+ cases, with over 90% of participants preferring the method for its comfort and ease [4]. Together, these findings support the Q-Pad as a promising, scalable alternative for HR-HPV screening, particularly in settings where traditional approaches are less accessible.
References
[1] Human Papillomavirus DNA Detection in Menstrual Blood from Patients with Cervical Intraepithelial Neoplasia and Condyloma Acuminatum, Wong SC et al., Journal of Clinical Microbiology, 2010.
[2] Feasibility and Accuracy of Menstrual Blood Testing for High-risk Human Papillomavirus Detection With Capture Sequencing, Zhang J et al., JAMA Network Open, 2021.
[3] Detection of High-Risk Human Papillomavirus Using Menstrual Blood in Women With High-Grade Squamous Intraepithelial Lesions: A Pilot Study, Lee B et al., Journal of Obstetrics and Gynaecology Research, 2016.
[4] Screening for High-Risk Human Papillomavirus Using Passive, Self-Collected Menstrual Blood, Naseri S et al., Obstetrics & Gynecology, 2022.
JOIN US
Cervical cancer is entirely preventable
with early detection.
Be a part of the solution and solve cervical cancer.
Funding
If you represent a diagnostic platform, healthcare organization, or country-level agency and would like to work with us, please fill out this form and we will get back to you.
Research
If you are a scientist, foundation, or non-profit interested in collaborating on studies or supporting our innovation, please fill out the form and we will get back to you.
